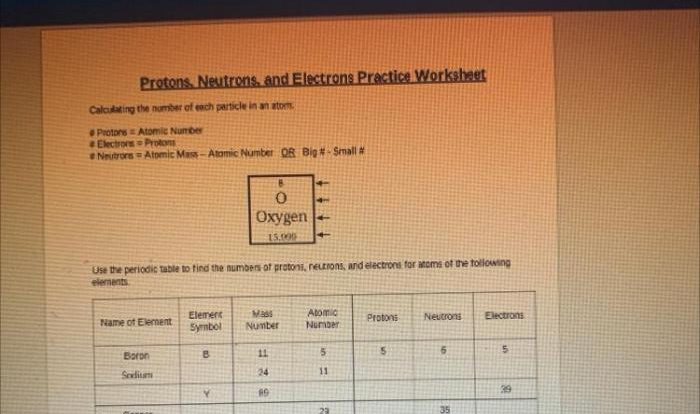
Rank the following elements in order of decreasing atomic radius: hydrogen, helium, lithium, beryllium, boron, carbon, nitrogen, oxygen, fluorine, and neon. This question delves into the fascinating world of atomic structure, where we explore the fundamental properties that govern the behavior of matter.
Atomic radius, a crucial concept in chemistry, refers to the distance from the nucleus to the outermost electron shell of an atom. Understanding atomic radius is essential for comprehending chemical bonding, crystal structures, and the physical properties of elements.
Elements and Atomic Radius
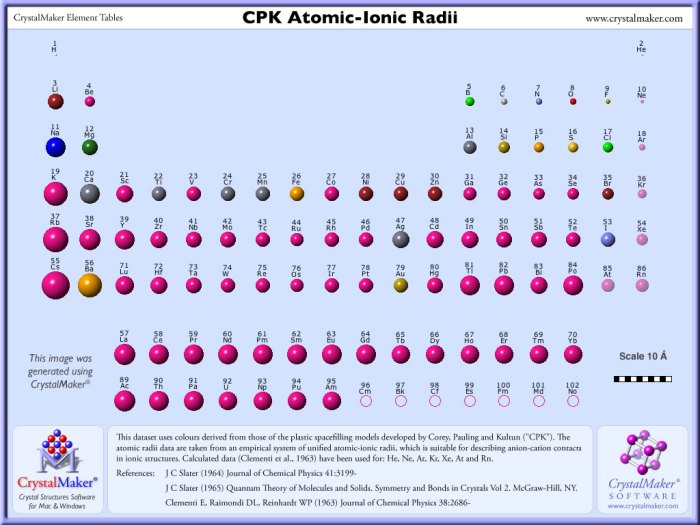
Atomic radius is a measure of the distance from the nucleus to the outermost electron shell of an atom. It is an important property that influences many chemical and physical properties of elements.
The atomic radii of the following elements are:
| Element | Atomic Radius (pm) |
|---|---|
| Hydrogen | 53 |
| Helium | 31 |
| Lithium | 155 |
| Beryllium | 111 |
| Boron | 85 |
| Carbon | 70 |
| Nitrogen | 65 |
| Oxygen | 60 |
| Fluorine | 50 |
| Neon | 38 |
Factors Affecting Atomic Radius
Several factors affect the atomic radius of an element:
Number of Electron Shells
As the number of electron shells increases, the atomic radius generally increases. This is because the outermost electrons are further away from the nucleus, which has a stronger attraction for the electrons in the inner shells.
Nuclear Charge
The nuclear charge is the number of protons in the nucleus. As the nuclear charge increases, the attraction between the nucleus and the electrons increases, which pulls the electrons closer to the nucleus and reduces the atomic radius.
Shielding Effect
The shielding effect refers to the ability of inner electrons to reduce the attraction between the nucleus and the outermost electrons. As the number of inner electrons increases, the shielding effect increases, which weakens the attraction between the nucleus and the outermost electrons and increases the atomic radius.
Trends in Atomic Radius: Rank The Following Elements In Order Of Decreasing Atomic Radius
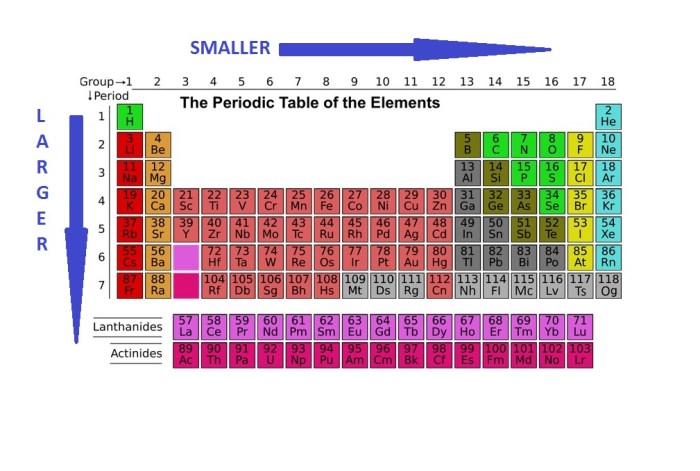
There are two main trends in atomic radius across periods and groups in the periodic table:
Across Periods, Rank the following elements in order of decreasing atomic radius
Moving from left to right across a period, the atomic radius generally decreases. This is because the nuclear charge increases across a period, while the number of electron shells remains the same. The increasing nuclear charge pulls the electrons closer to the nucleus, reducing the atomic radius.
Down Groups
Moving down a group, the atomic radius generally increases. This is because the number of electron shells increases down a group, while the nuclear charge remains relatively constant. The additional electron shells push the outermost electrons further away from the nucleus, increasing the atomic radius.
The following graph shows the trend in atomic radius across Period 2 elements:

Applications of Atomic Radius
Knowledge of atomic radius is useful in understanding various chemical and physical properties of elements:
Chemical Bonding
Atomic radius influences the type and strength of chemical bonds formed by an element. Elements with smaller atomic radii tend to form stronger bonds, while elements with larger atomic radii tend to form weaker bonds.
Crystal Structures
Atomic radius determines the arrangement of atoms in crystal structures. Elements with similar atomic radii tend to form crystals with similar structures.
Physical Properties of Elements
Atomic radius affects the physical properties of elements, such as density, melting point, and boiling point. Elements with smaller atomic radii tend to be denser, have higher melting points, and higher boiling points.
FAQ Explained
What is the relationship between atomic radius and the number of electron shells?
As the number of electron shells increases, the atomic radius generally increases. This is because the outermost electrons are located further from the nucleus, resulting in a larger atomic size.
How does nuclear charge affect atomic radius?
A higher nuclear charge attracts the electrons more strongly, pulling them closer to the nucleus. This results in a smaller atomic radius.
What is the shielding effect?
The shielding effect refers to the ability of inner electrons to reduce the attraction between the nucleus and the outermost electrons. This effect weakens the pull of the nucleus on the outermost electrons, leading to a larger atomic radius.